1353
Views & Citations353
Likes & Shares
Aptamers are oligonucleotide (DNA/RNA) or peptide molecules specialized in binding to specific targets including cells, nucleotides, proteins, peptides, antibiotics and other small molecules. Due to small size and highly specificity nature, aptamers can penetrate tissue barriers efficiently and move easily inside the target cells. They can improve tumor-to-blood and tumor-to normal tissue ratios and directly contribute towards therapeutic indices. Aptamers are a well-known tool, which is involved in various clinical applications such as cancer, virus and bacterial infection detection. In addition to this, aptamers are also helpful in food industries for contaminant detection and pesticide detection in the field of agriculture. SELEX (Systemic Evolution of Ligands by EXponential enrichment) is a novel technique to design the potent aptamer sequences. However, aptamers are an important diagnostic tool but still various stochastic and empirical studies are required to design the novel aptamers that can significantly improve the diagnosis of life threatening viruses.
Keywords: DNA, RNA, Protein, SELEX, Cancer, Diagnostic, Therapeutic
Aptamers are oligonucleotide (DNA/RNA) or peptide molecules specialized in the specific and efficient binding to a target molecule like cells, nucleotides, proteins, peptides, antibiotics and other small molecules [1]. They have various advantages over antibodies, because of their small size, easy modification and higher stability in physical and chemical environments. Novel aptamers are highly specific to their targets even in life threatening diseases such as cancerous cells, bacterial and viral infectious cells [2-9]. They can efficiently penetrate tissue barriers and easily move inside the target cells, could improve tumor-to-blood and tumor-to normal tissue ratios and directly contributing towards therapeutic indices [10]. Due to low cost production, highly sensitive, nontoxic and low immunogenicity of aptamer [11], it could be more prominent for clinical and industrial applications than monoclonal antibodies. Aptamers are thermostable, which allows their easy storage and transportation. The first approved aptamer by FDA (Food and Drug Administration) was named as Pegaptanib and is delivered by Pfizer under the name Macugen. It is a PEGylated RNA aptamer, a fluoromodified sugar used to treat macula degeneration [12]. Some of the commercially available aptasensors which are used for clinical diagnosis are listed in Table 1.
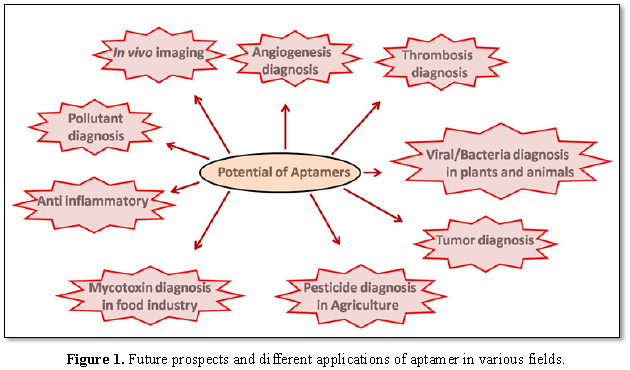
Systemic evolution of ligands by exponential enrichment (SELEX) is a combinatorial chemistry technique of molecular biology, designed to identify aptamer sequences [13,14]. It is reported that RNA aptamers are commonly used for therapies whereas DNA aptamers can be used as diagnostic tools. Aptamers are widely being studied as a biomaterial in various investigations pertaining to their use as a bio-sensing tool, diagnostic tool and therapeutic tool and also for the development of new drugs (Figure 1) [15]. In recent studies, aptamers inhibit the action of botulinum neurotoxin (a poison for mankind) [16]. They have been reported to be potential antidotes to treat deadly botulism [17]. A scientist named Frith has developed a highly potent aptamer, which is specific against P. falciparum [18]. Aptamers prove to be a critical vehicle to accomplish explicit medication conveyance to malignant growth cells. Presently, numerous cancer stem cell targeting aptamers have been created and aptamer-based nano-drug frameworks have accomplished anticancer stem cells impacts in preclinical examinations [19].
An aptamer, termed as E3, has been identified, which selectively penetrates only prostate cancerous cells. It also target ovarian, melanoma, breast, colon, lung, leukemia, liver, glioblastoma cancers in addition to prostate cancer. It was reported that linking E3 aptamer to a Pseudomonas exotoxin (PE-LR-8) killed the prostate malignant growth cells in vitro with no side effects towards healthy cells [20]. It was also found that linking of E3 bound to the lethal monomethylauristatin drugs can kill the malignant prostate cells in vitro [21]. Aptamers can be used as agonists as well as antagonists to target the receptors on cells with high specificity and affinity [22]. It also characterized the RNA aptamer against a biomarker, expressed on germinal center B cells and chronic myelogenous leukemias [23].
Health problems are increasing worldwide due to the allergens, pathogens and bio toxins present in food and pharmacological residues or the contaminants of industrial origin (such as packaging materials, pesticides, dyes or adulterants) that can migrate into food products [24]. There is an increasing demand for sensitive, rapid, easy to use, specific and cost effective methods to detect such contaminants in food. Biosensors using aptamers as recognition elements are termed as aptasensors which were designed to detect the contaminants in food (Figure 1) [25]. Several biosensors were designed to check the toxic elements in milk samples and food packaging materials [26,27]. It was observed that novel aptamers could be helpful in the field of agriculture also, for the detection of extensively used pesticides (acetamiprid and atrazine) (Figure 1) [28].
FUTURE PROSPECTIVES
Aptamers have great potential in the development of microarrays studies. One of the major advantages of aptamers-based therapy is capability of selective targeting against cancerous malignant cells whereas other non-aptamers-based therapies kill the healthy cells with many adverse side effects (while using radiotherapy/chemotherapy) [29]. As aptamers can easily penetrate cell and organelles without immunogenic response, however it could be a better choice for advancement in tumor and metastasis treatment at divergent locations like circulation, cancerous cells, tumor stromal cells, associated vessels and pre-metastatic vascular niche. In future, aptamers could easily replace the monoclonal antibody for the detection of biological as well as non-biological contaminants from the agricultural as well as food materials. Furthermore, the stochastic and empirical studies could design the novel aptamers that significantly improve the diagnosis of life threatening viruses such as Ebola, HIV, ZIKA, Hepatitis and Rubella [30].
CONCLUSION
Since the use of aptamers is highly safe in diagnostics, as they show rare side effects against the health. Many studies have revealed the potential of aptamers in the area of therapeutic and diagnostic treatment, but there are still some stochastic parameters, which are unknown in the detection of severe viruses (rubella). A major challenge with aptamers is the requirement of different buffers, additives, ions, ion strengths and their optimum execution in a multiplex microarray for their proper folding and functioning to maintain their specificity. Taken together, aptamers are small in size, safe in use, synthesized within short periods and in high batches and highly specific in nature.
1. Jo H, Ban C (2016) Aptamer-nanoparticle complexes as powerful diagnostic and therapeutic tools. Exp Mol Med 48: 230.
2. Shum KT, Zhou J, Rossi JJ (2013) Aptamer-based therapeutics: New approaches to combat human viral diseases. Pharmaceuticals 6: 1507-1542.
3. Wandtke T, Woźniak J, Kopiński P (2015) Aptamers in diagnostics and treatment of viral infections. Viruses 7: 751-780.
4. Ciesiolka J, Gorski J, Yarus M (1995) Selection of an RNA domain that binds Zn2+. RNA: 538-550.
5. Yang Q, Goldstein IJ, Mei HY, Engelke DR (1998) DNA ligands that bind tightly and selectively to cellobiose. PNAS 95: 5462-5467.
6. Stoltenburg R, Nikolaus N, Strehlitz B (2012) Capture-SELEX: Selection of DNA aptamers for aminoglycoside antibiotics. J Anal Methods Chem.
7. Liu Z, Duan JH, Song YM, Ma J, Wang FD, et al. (2012) Novel HER2 aptamer selectively delivers cytotoxic drug to HER2-positive breast cancer cells in vitro. J Transl Med 10(1): 148.
8. Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, et al. (2006) Aptamers evolved from live cells as effective molecular probes for cancer study. PNAS 103: 11838-11843.
9. Liu Z, Duan JH, Song YM, Ma J, Wang FD, et al. (2012) Novel HER2 aptamer selectively delivers cytotoxic drug to HER2-positive breast cancer cells in vitro. J Transl Med 10: 148.
10. Xiang D, Shigdar S, Qiao G, Wang T, Kouzani AZ, et al. (2015) Nucleic acid aptamer-guided cancer therapeutics and diagnostics: The next generation of cancer medicine. Theranostics 5: 23-42.
11. Lao YH, Phua KK, Leong KW (2015) Aptamer nanomedicine for cancer therapeutics: Barriers and potential for translation. ACS Nano 9: 2235-2254.
12. Ng EW, Shima DT, Calias P, Cunningham ET, Guyer DR, et al. (2006) Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 5: 123.
13. Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249: 505-510.
14. Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346: 818.
15. Song KM, Lee S, Ban C (2012) Aptamers and their biological applications. Sensors 12: 612-631.
16. Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, et al. (2001) Botulinum toxin as a biological weapon: Medical and public health management. JAMA 285: 1059-1070.
17. Chang TW, Blank M, Janardhanan P, Singh BR, Mello C, et al. (2010) In vitro selection of RNA aptamers that inhibit the activity of type Abotulinum neurotoxin. Biochem Biophys Res Commun 396: 854-860.
18. Frith KA, Fogel R, Goldring JD, Krause RG, Khati M, et al. (2018) Towards development of aptamers that specifically bind to lactate dehydrogenase of plasmodium falciparum through epitopic targeting. Malar J 17: 191.
19. Famulok M, Hartig JS, Mayer G (2007) Functional aptamers and aptazymes in biotechnology, diagnostics and therapy. Chem Rev 107: 3715-3743.
20. Gray BP, Kelly L, Levy M, Sullenger BA (2015) A novel aptamer targeting agent for prostate cancer. Mol Ther 23: 28-29.
21. Katz E, Willner I (2003) Probing biomolecular interactions at conductive and semi conductive surfaces by impedance spectroscopy: Routes to impedimetric immunosensors, DNA‐sensors and enzyme biosensors. Electroanal 15: 913-947.
22. Coiffier B, Lepage E, Herbrecht R, Tilly H, Solal-Celigny P, et al. (2000) MabThera (rituximab) plus CHOP is superior to CHOP alone in elderly patients with diffuse large B-cell lymphoma (DLCL): Interim results of a randomized GELA trial. Blood 96: 223-223.
23. Pratico ED, Nair SK, Sullenger BA (2015) Identification and characterization of a B cell aptamer that targets diffuse large B cell lymphoma (DLBCL) and chronic myelogenous leukemia (CML). Mol Ther 23: 29.
24. Adley CC (2014) Past, present and future of sensors in food production. Foods 3: 491-510.
25. Amaya-González S, de-los-Santos-Álvarez N, Miranda-Ordieres AJ, Lobo-Castañón MJ (2013) Aptamer-based analysis: A promising alternative for food safety control. Sensors 13: 16292-16311.
26. Dudak FC, Boyaci İH (2014) Peptide-based surface plasmon resonance biosensor for detection of staphylococcal enterotoxin b. Food Anal Methods 7: 506.
27. Yang J, Kim SE, Cho M, Yoo IK, Choe WS, et al. (2014) Highly sensitive and selective determination of bisphenol-A using peptide-modified gold electrode. Biosens Bioelectron 61: 38-44.
28. Madianos L, Tsekenis G, Skotadis E, Patsiouras L, Tsoukalas D (2018) A highly sensitive impedimetric aptasensor for the selective detection of acetamiprid and atrazine based on micro wires formed by platinum nanoparticles. Biosens Bioelectron 101: 268-274.
29. Tang Z, Shangguan D, Wang K, Shi H, Sefah K, et al. (2007) Selection of aptamers for molecular recognition and characterization of cancer cells. Anal Chem 79: 4900-4907.
30. Molefe PF, Masamba P, Oyinloye BE, Mbatha LS, Meyer M, et al. (2018) Molecular application of aptamers in the diagnosis and treatment of cancer and communicable diseases. Pharm 11: 93.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Dermatology Clinics and Research (ISSN:2380-5609)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Spine Diseases
- Oncology Clinics and Research (ISSN: 2643-055X)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)